Chronic kidney disease products and services provider Fresenius Renal Therapies has unveiled the latest technological upgrade to its Crit-Line product line, the CLiC device. An estimated 2.8 million patients with kidney disease worldwide regularly undergo dialysis treatment — a blood cleansing procedure that substitutes for kidney function cases of failure of the vital organ.
Chronic kidney disease products and services provider Fresenius Renal Therapies has unveiled the latest technological upgrade to its Crit-Line product line, the CLiC device. An estimated 2.8 million patients with kidney disease worldwide regularly undergo dialysis treatment — a blood cleansing procedure that substitutes for kidney function cases of failure of the vital organ.
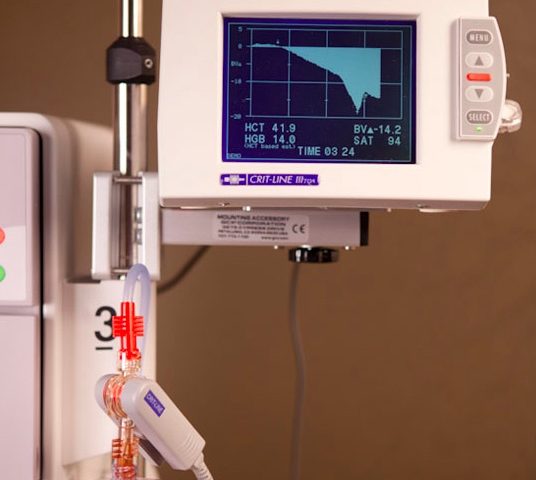
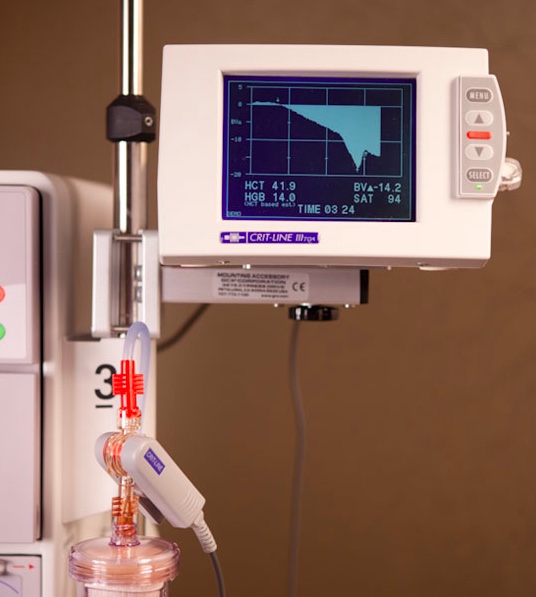
The Crit-Line blood chamber is a sterile, single use, disposable, optical cuvette designed for use with the Crit-Line sensor clip during acute and chronic hemodialysis therapy. The blood chamber is connected between the arterial bloodline and the dialyzer within the extracorporeal circuit during the hemodialysis treatment. The monitor allows continuous, non-invasive measurement of a dialysis patient’s hematocrit (percentage of whole blood volume made up of red blood cells) and oxygen saturation (the fraction of oxygen-saturated hemoglobin relative to total hemoglobin) in real-time during hemodialysis (HD) treatment.
In addition, the Crit-Line device calculates percentages of change in a dialysis patient’s intravascular blood volume. The device’s sensor clip measures hematocrit, percent change in blood volume and oxygen saturation in real time, and based on data the monitor provides, the clinician/nurse, under physician direction, is enabled to make real-time interventions during hemodialysis (i.e. increasing or decreasing the rate at which fluid is removed from the blood) in order to optimize removal of the maximum amount of fluid at the most tolerable rate for the patient consistent with preventing common dialysis-associated symptoms of such as nausea, cramping and vomiting, thereby helping caregivers deliver more effective dialysis treatment.
The Bad Homburg v.d.H., Germany headquartered company, its North American division Fresenius Medical Care North America (FMCNA) located in Waltham, Massachusetts, notes that as an integrated feature of its flagship HD delivery system — the 2008T hemodialysis machine — the Crit-Line monitor CLiC device provides healthcare professionals with immediate, real-time access to a patient’s blood status during HD treatment, as well as essential information needed to make informed intradialytic adjustments. They observe that fluid overload is a common and serious problem that can result in severe complications, and is a major cause of hospitalizations in the dialysis patient populations, and that bringing fluid overload under control in these patients has been a central focus of clinicians and policy makers for many years.
 “We have been looking forward to the release of CLiC technology”, says Mark Costanzo, FMCNA Renal Therapies Group President in a release. “Fluid overload in dialysis patients is an important issue that needs to be addressed’, he continues. “I’m proud to support the introduction of a technological breakthrough that will allow nephrologists to have access to important data and make informed decisions that will directly impact the health and well-being of this chronically-ill population. Fresenius Renal Therapies is dedicated to working with nephrologists and dialysis clinicians in a continued effort to improve patient outcomes and overall quality of life”.
“We have been looking forward to the release of CLiC technology”, says Mark Costanzo, FMCNA Renal Therapies Group President in a release. “Fluid overload in dialysis patients is an important issue that needs to be addressed’, he continues. “I’m proud to support the introduction of a technological breakthrough that will allow nephrologists to have access to important data and make informed decisions that will directly impact the health and well-being of this chronically-ill population. Fresenius Renal Therapies is dedicated to working with nephrologists and dialysis clinicians in a continued effort to improve patient outcomes and overall quality of life”.
 “The CLiC device represents a significant step forward in the evolution of volume management”, says FMCNA Chief Medical Officer Frank Maddux, MD. “By assisting clinical staff in managing hemodialysis patients complex fluid and intravascular volume conditions, the CLiC will be a valuable tool for clinicians. Moreover, with the integration of the CLiC device into the 2008T machine, clinical staff can more quickly recognize a patient’s risk for low blood pressure during hemodialysis resulting from certain ultrafiltration and intravascular blood volume characteristics. Once the CLiC device alerts staff, they can pause ultrafiltration until the clinicians can assess the situation of their patient. This industry-leading device is a significant step forward in improving patients quality of life”.
“The CLiC device represents a significant step forward in the evolution of volume management”, says FMCNA Chief Medical Officer Frank Maddux, MD. “By assisting clinical staff in managing hemodialysis patients complex fluid and intravascular volume conditions, the CLiC will be a valuable tool for clinicians. Moreover, with the integration of the CLiC device into the 2008T machine, clinical staff can more quickly recognize a patient’s risk for low blood pressure during hemodialysis resulting from certain ultrafiltration and intravascular blood volume characteristics. Once the CLiC device alerts staff, they can pause ultrafiltration until the clinicians can assess the situation of their patient. This industry-leading device is a significant step forward in improving patients quality of life”.
For customers utilizing other 2008 series machines, FMCNA has introduced the Crit-Line IV monitor, a stand-alone device providing access to patient profile information, including hematocrit, oxygen and percent change in intravascular blood volume during HD treatment.
FMCNA operates 40 production sites on all continents globally providing dialysis products such as dialysis machines, dialyzers and related disposables. In the U.S., the company maintains facilities for development of devices in Concord and Lake Forest, California, and for dialyzers and other disposable products in Ogden, Utah.
For more information, visit the FMCNA website at:
http://www.freseniusmedicalcare.us
For information about Crit-Line Technology visit:
http://www.fmcna-crit-line.com
Sources:
Fresenius Renal Therapies
Fresenius Medical Care North America (FMCNA)